We have all heard the story that exercise is healthy for you; there is a bit of a logical disconnect here – exercise is one of the greatest stressors that occur in our daily lives; there is trauma to our joints, stress on our heart, oxygen deprivation to our tissues, huge increases in inflammation. And this is healthy? This will help us live longer? I thought that a major part of aging and disease was stress and inflammation? How does this make sense?
Theory of Aging & Exercise

Aging is a part of life, it may be natural, healthy is another issue. It involves a complicated process of time-dependent progressive loss of physical and cognitive functioning paired with cellular damage accumulated across the lifespan. Within the next two decades, the older population will nearly double in size making the study of aging and how to stall the negative aspects of a critical research topic.
Two main theories of aging explain the process as a combination of programmed aging and damage or error on the biological level. Mostly, there is a finite amount of time that cells can survive and cumulative damage incurred from life choices can increase or decrease the speed of cellular damage. Those that prescribe to the programmed aspect of aging believe that the body is not only programmed to deteriorate but also to exert less control over the errors and damages that occur.
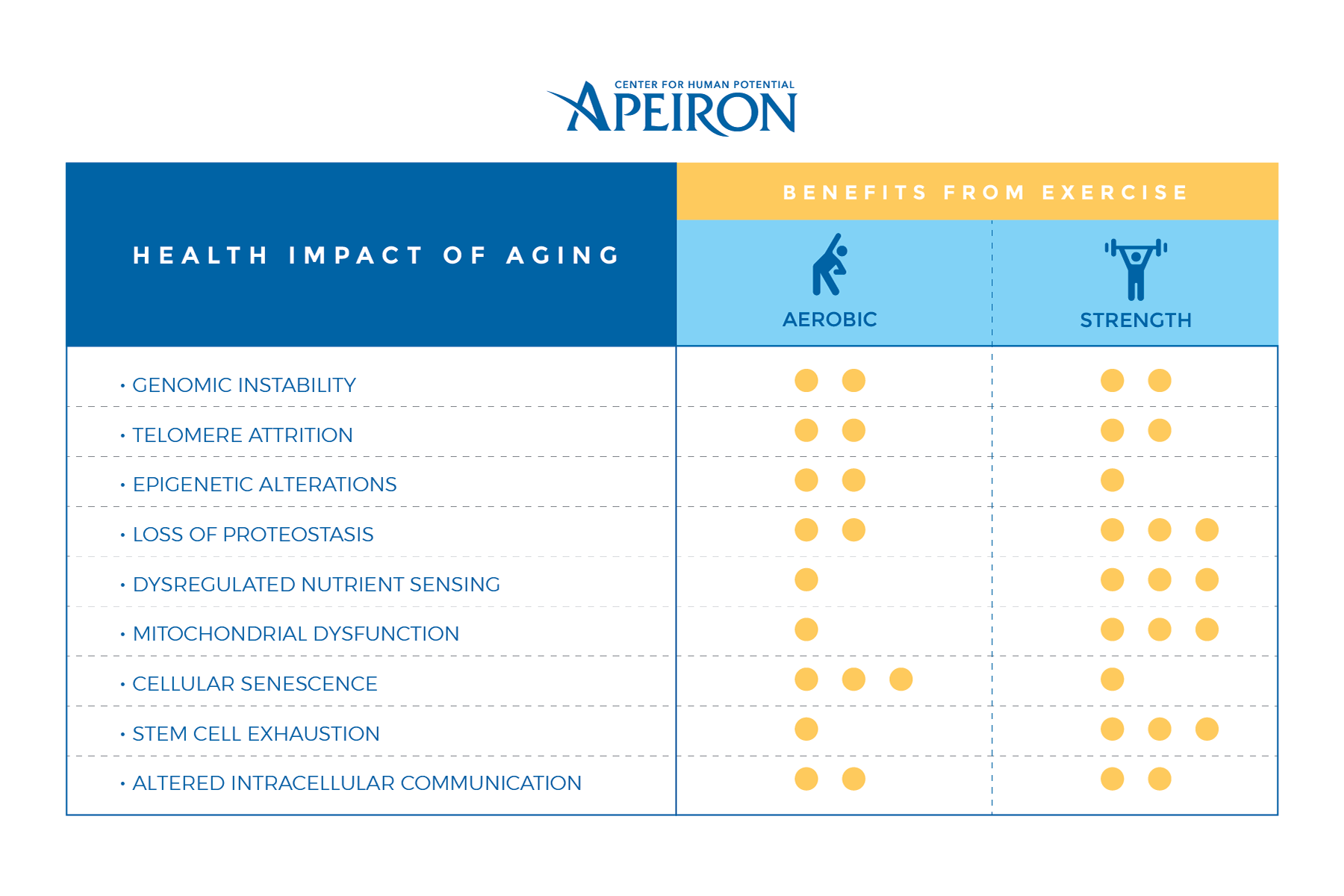
The aging process is comprised of nine different hallmarks including; genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulation nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.
Aging has been associated with chronic cardiovascular, cardiorespiratory, musculoskeletal, and cognitive disorders. Physical activity is known to prevent muscle atrophy, improve or boost metabolic activity, improve respiratory function, and positively impact muscles regarding strength, endurance, and stability. The positive benefits or physical activity can even be seen on the cellular level.
Genomic Instability
The accumulation of cellular damage over the lifespan is known as genomic instability. The damage can be caused by exogenous or endogenous factors, cause mutations, gene disruption, telomere shortening, and chromosomal gains or losses. As those damages accumulate, the aging processes are sped up.

Exercise has been linked to genomic stability. Aerobic and strength training can improve DNA repair mechanisms and reduce the incidence of DNA methylation which in turn reduces risk factors for various chronic conditions including heart disease and cancer.
Telomere Attrition
Telomeres are protein structures that protect the integrity of DNA. The length of telomeres naturally decreases with age and time until it reaches a minimum critical size and the DNA is no longer functional. Because telomere length decreases with age it has been viewed as a biological marker for aging and telomere length in adults is associated with the number of healthy living years the individual has left.
Telomere length can be increased and thus in a sense slow or reverse some aspects of aging.
Physical activity increases the up-regulation of protective proteins and DNA repair which lengthens telomeres. Click To TweetIt also helps protect telomeres from stress-related damage.
Epigenetic Alterations
Epigenetic changes are alterations of the DNA as a result of natural aging, environment, behavior, nutrition, mental factors, and physical exercise. Several members of the sirtuin (SIRT) family (SIRT1, SIRT3, and SIRT6) contribute to healthy aging, and reduced functioning of this family can reduce the longevity of the DNA.
Aerobic exercise can positively influence epigenetic alterations of DNA. Click To TweetRegular exercise improves functioning through DNA methylation, up-regulate brain-derived neurotrophic factor (BDNF), promote gene remodeling, and combat frailty. Acute exercise increases mRNA expression enabling protein synthesis and structural remodeling.
Loss of Proteostasis
Natural aging and some age-related diseases can impair protein homeostasis in DNA. Loss of these proteins can contribute to neurological deficits connected to Alzheimer’s and Parkinson’s disease.
Physical activity induces autophagy in the brain and muscles. This decreases risk for various chronic diseases by eliminating damaging proteins, improving strength, and increasing muscle mass.
Deregulated Nutrient Sensing
Growth hormone (GH) is produced in the pituitary gland. During the third decade of life, GH begins to reduce along with insulin-like growth factor (IGF-1) progressively. As a result, aging is sped up due to the limited capacity of cells and DNA to repair themselves and grow.
Resistance and high-intensity training can both promote protein synthesis. These forms of exercise can improve an anabolic cellular rate which increases GH and IGF-1 at speeds equivalent to the intensity and duration of the exercise regimen.
Mitochondrial Dysfunction
During the aging process, mitochondrial integrity is impaired on multiple domains. Free radicals can cause damage to DNA, mtDNA mutations can develop, and oxidative stress and further damage DNA. This type of dysfunction can occur in various systems within the body including the nervous, muscular, and skeletal systems. The damage accumulates over time and can result in physiologic damage.
Regular exercise can play a crucial role in increasing the available pool of functional mitochondria. Resistance training program can, is regularly used, reverse some aging-related dysfunction to a state similar to younger adults.
Cellular Senescense
When cells reach their limited ability to regenerate, it is known as cellular senescence. The rate of cellular senescence is not stable across all type of cell tissue in the body which can result in faster or slower aging of different body systems.
Aerobic exercise can promote increased levels of natural killer cells which decrease risks of cancer and improved recovery rates from cancer, result in up-regulation of cardiac stabilizing proteins, reduce vascular expression of apoptosis regulators, and reduce DNA damage biomarkers.
Stem Cell Exhaustion
There are a limited number of stem cells available in the body, and as they are used, they can’t be replaced. As a result, there is a reduced capacity to regenerate cells. Satellite cells are the most important in the management and repair of type II muscle fibers.
Resistance training can be used in young adults through the aging process to aid in skeletal muscle regeneration through proliferation and differentiation of satellite cells – which are essentially the stem cells of our muscle.
Altered Intercellular Communication
Cellular communication in neuroendocrine, endocrine, and neuronal levels becomes impaired during aging. Altered communication leads to a pro-inflammatory state known as inflammaging where hypothalamic NF-kB expression is activated, the gonadotropin-releasing hormone is downregulated, and there is an increased risk for bone frailty and muscle weakness.
Chronic exercise can lead to restorative effects which lower inflammation levels through decreased levels of C-reactive protein and IL-6. Click To TweetConclusions
Practicing regular exercise can lead to an increased quality of life, improved functional capacity, and more physical independence. A combination of strength training and aerobic exercise of increasing intensity should be used to maximize the potential of cellular and DNA benefits. Individuals with chronic conditions may need to take a modified approach to exercise, but any movement is beneficial for sedentary people.
Starting an exercise regimen or determining the best fit for your life and health can seem daunting. A coach can help examine ways for you to maximize physical activity in your life and reap the benefits of movement on DNA to slow and reverse the effects of aging.








